- Do you have the right policies and procedures in place to develop medical device software?
- Do you have an appropriate quality management system established for developing software that is considered a medical device?
- Do you have a robust software development lifecycle with processes established that ensure quality and patient safety?
- Do you have appropriate and effective risk management processes established?
- Do you know what regulations and guidance are required to meet the requirements for bringing Medical Device Software to market?
- Are you utilizing best practices for developing Medical Device Software?
- Does your organization maintain a Culture of Quality and Organizational Excellence that meets the expectations of the regulatory
precertification program?
SERVICES WE PROVIDE:
- Medical Device Software Lifecycle Processes
Establish the required Medical Device Software life cycle processes to maintain regulatory compliance.
- Compliance Risk Assessments
Perform compliance risk assessments against the applicable regulations to identify potential gaps and risks the client may
have within their current practices for developing Medical Device Software.
- Optimization and Improvement
Drive greater effciency in the development practices for developing Medical Device Software to reduce cycle time.
- Risk Management Process
Implement effective risk management processes for developing Medical Device Software.
- Outsourcing Medical Device Software Development
Perform supplier assessments for our clients who are outsourcing their Medical Device Software development to ensure
regulatory requirements are met.
- Independant Software Design Review
Conduct independent software design reviews through the critical points within the development life cycle.
- Software Maintenence
Establish solid processes for maintaining software during post market surveillance.
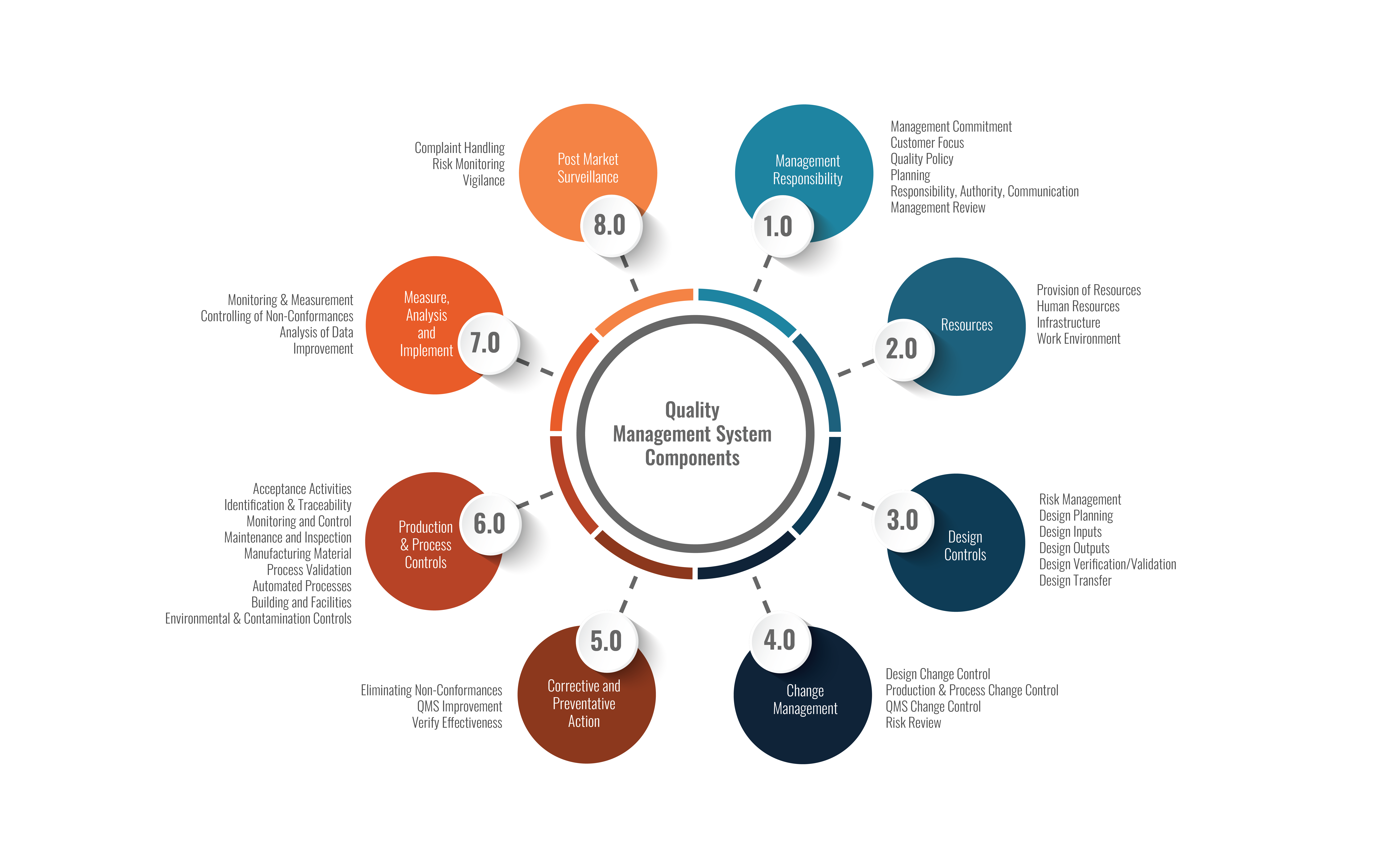
- Quality Management System
Implementing and improving the quality management system to support the Medical Device Software and Total Product
Life Cycle processes. (See graph below:)
Navigating the Regulatory Environment with Ever-Changing Advancements in Technology
Software and artificial intelligence are being utilized to dramatically improve and innovate patient health. However, increased controls that mitigate patient risk are now required. Controls must ensure that the benefits outweigh the associated risks. The accelerated advancement of technology has brought both benefits as well as increased challenges in regards to patient safety. It is critical to have the appropriate controls during the entire product life cycle. In addition, navigating the regulatory environment can be very challenging when the regulatory bodies are also trying to stay up-to-date with the latest technological advances, with their focus on bringing new solutions quickly to market that benefit patients and ensure safety.
